In Stock
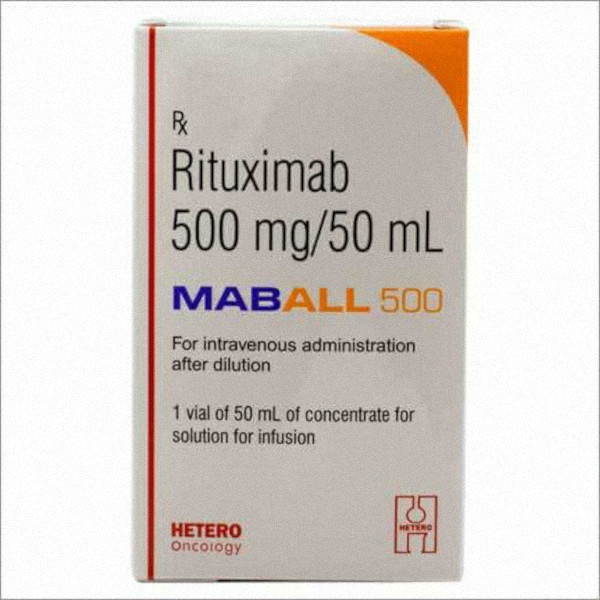
Maball (generic rituximab) 1 vial
- Identical in effectiveness to brand name original
- Produced by a reputable pharmaceuticals company
- Monoclonal antibody medication
- 500 mg/50 mL dosage
$1,400.00
Rituximab is a monoclonal antibody medication used to treat certain autoimmune diseases and some types of cancer.
It is a chimeric monoclonal antibody targeted against CD20 which is a surface antigen present on B cells. Therefore, it acts by depleting normal as well as pathogenic B cells while sparing plasma cells and hematopoietic stem cells as they do not express the CD20 surface antigen.
In the United States, rituximab is indicated to treat:
- Non-Hodgkin lymphoma (NHL)
- Chronic lymphocytic leukemia (CLL)
- Rheumatoid arthritis having inadequate response to one or more TNF inhibitors
- Vasculitis such as granulomatosis with polyangiitis and microscopic polyangiitis
- Moderate to severe pemphigus vulgaris
- In combination with chemotherapy for children (≥6 months to <18 years) with previously untreated, advanced stage, CD20-positive diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), Burkitt-like lymphoma (BLL), or mature B-cell acute leukemia (B-AL)
Rituximab has been shown to be an effective rheumatoid arthritis treatment in three randomised controlled trials and is now licensed for use in refractory rheumatoid disease.
In the United States, it has been FDA-approved for use in combination with methotrexate (MTX) for reducing signs and symptoms in adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more anti-TNF-alpha therapy.
In the European Union, the license is slightly more restrictive: it is licensed for use in combination with MTX in patients with severe active RA who have had an inadequate response to one or more anti-TNF therapy.